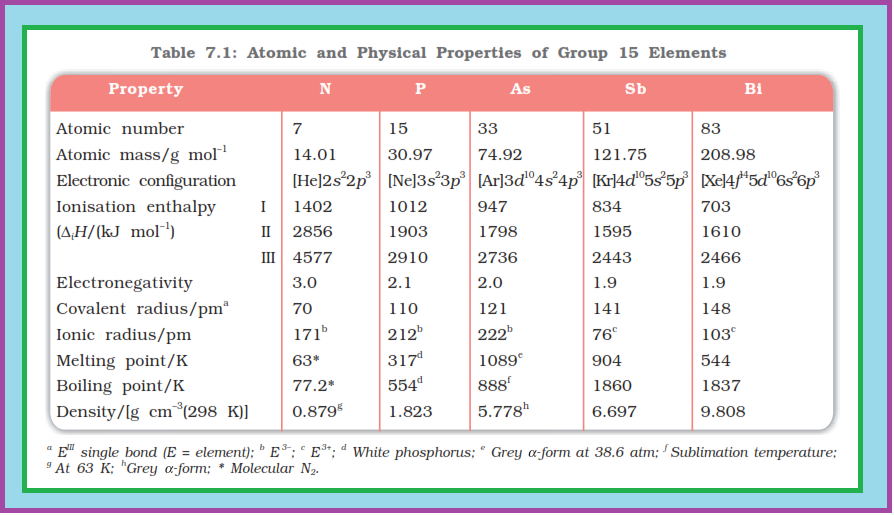
`=>` Nitrogen differs from the rest of the members of this group due to its smaller size, high electronegativity, high ionisation enthalpy and non-availability of `d`-orbitals.
`=>` Nitrogen has unique ability to form `pπ -pπ` multiple bonds with itself and with other elements having small size and high electronegativity (e.g., `C`, `O`).
`=>` Heavier elements of this group do not form `pπ -pπ` bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping.
`=>` Thus, nitrogen exists as a diatomic molecule with a triple bond (one `s` and two `p`) between the two atoms. Consequently, its bond enthalpy (941.4 kJ mol`text()^(–1)`) is very high.
`=>` On the contrary, phosphorus, arsenic and antimony form single bonds as `P–P`, `As–As` and `Sb–Sb` while bismuth forms metallic bonds in elemental state.
`=>` However, the single `N–N` bond is weaker than the single `P–P` bond because of high interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen.
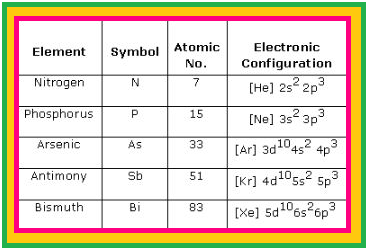
`=>` Another factor which affects the chemistry of nitrogen is the absence of `d` orbitals in its valence shell.
`=>` Besides restricting its covalency to four, nitrogen cannot form `dπ –pπ` bond as the heavier elements can e.g., `color{red}(R_3P = O)` or `color{red}(R_3P = CH_2)` (`R` = alkyl group).
`=>` Phosphorus and arsenic can form `dπ –dπ` bond also with transition metals when their compounds like `color{red}(P(C_2H_5)_3)` and `color{red}(As(C_6H_5)_3)` act as ligands.
(i) `color{green}("Reactivity towards hydrogen ")` All the elements of Group 15 form hydrides of the type `color{red}(EH_3)` where `color{red}(E = N, P, As, Sb)` or `color{red}(Bi)`.
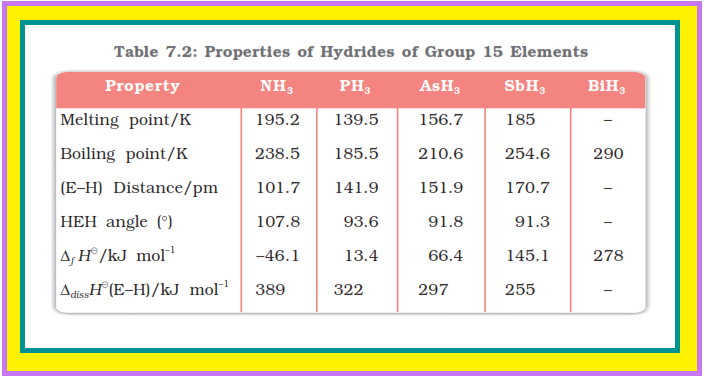
● Some of the properties of these hydrides are shown in Table 7.2.
● The hydrides show regular gradation in their properties.
● The stability of hydrides decreases from `color{red}(NH_3)` to `color{red}(BiH_3)` which can be observed from their bond dissociation enthalpy.
● As a result, the reducing character of the hydrides increases.
● Ammonia is only a mild reducing agent while `color{red}(BiH_3)` is the strongest reducing agent amongst all the hydrides.
● Basicity also decreases in the order `color{red}(NH_3 > PH_3 > AsH_3 > SbH_3 le BiH_3)`.
(ii) `color{green}("Reactivity towards Oxygen ")` All these elements form two types of oxides : `color{red}(E_2O_3)` and `color{red}(E_2O_5)`.
● The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state.
● Their acidic character decreases down the group.
● The oxides of the type `color{red}(E_2O_3)` of nitrogen and phosphorus are purely acidic, that of arsenic and antimony amphoteric and those of bismuth is predominantly basic.
(iii) `color{green}("Reactivity towards Halogens" )` : These elements react to form two series of halides : `color{red}(EX_3)` and `color{red}(EX_5)`.
● Nitrogen does not form pentahalide due to non-availability of the `d` orbitals in its valence shell.
● Pentahalides are more covalent than trihalides.
● All the trihalides of these elements except those of nitrogen are stable.
● In case of nitrogen, only `color{red}(NF_3)` is known to be stable.
● Trihalides except `BiF_3` are predominantly covalent in nature.
(iv) `color{green}("Reactivity towards Metals ")` : All these elements react with metals to form their binary compounds exhibiting `–3` oxidation state, such as, `color{red}(Ca_3N_2)` (calcium nitride) `color{red}(Ca_3P_2)` (calcium phosphide), `color{red}(Na_3As_2)` (sodium arsenide), `color{red}(Zn_3Sb_2)` (zinc antimonide) and `color{red}(Mg_3Bi_2)` (magnesium bismuthide).
`=>` Nitrogen differs from the rest of the members of this group due to its smaller size, high electronegativity, high ionisation enthalpy and non-availability of `d`-orbitals.
`=>` Nitrogen has unique ability to form `pπ -pπ` multiple bonds with itself and with other elements having small size and high electronegativity (e.g., `C`, `O`).
`=>` Heavier elements of this group do not form `pπ -pπ` bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping.
`=>` Thus, nitrogen exists as a diatomic molecule with a triple bond (one `s` and two `p`) between the two atoms. Consequently, its bond enthalpy (941.4 kJ mol`text()^(–1)`) is very high.
`=>` On the contrary, phosphorus, arsenic and antimony form single bonds as `P–P`, `As–As` and `Sb–Sb` while bismuth forms metallic bonds in elemental state.
`=>` However, the single `N–N` bond is weaker than the single `P–P` bond because of high interelectronic repulsion of the non-bonding electrons, owing to the small bond length. As a result the catenation tendency is weaker in nitrogen.
`=>` Another factor which affects the chemistry of nitrogen is the absence of `d` orbitals in its valence shell.
`=>` Besides restricting its covalency to four, nitrogen cannot form `dπ –pπ` bond as the heavier elements can e.g., `color{red}(R_3P = O)` or `color{red}(R_3P = CH_2)` (`R` = alkyl group).
`=>` Phosphorus and arsenic can form `dπ –dπ` bond also with transition metals when their compounds like `color{red}(P(C_2H_5)_3)` and `color{red}(As(C_6H_5)_3)` act as ligands.
(i) `color{green}("Reactivity towards hydrogen ")` All the elements of Group 15 form hydrides of the type `color{red}(EH_3)` where `color{red}(E = N, P, As, Sb)` or `color{red}(Bi)`.
● Some of the properties of these hydrides are shown in Table 7.2.
● The hydrides show regular gradation in their properties.
● The stability of hydrides decreases from `color{red}(NH_3)` to `color{red}(BiH_3)` which can be observed from their bond dissociation enthalpy.
● As a result, the reducing character of the hydrides increases.
● Ammonia is only a mild reducing agent while `color{red}(BiH_3)` is the strongest reducing agent amongst all the hydrides.
● Basicity also decreases in the order `color{red}(NH_3 > PH_3 > AsH_3 > SbH_3 le BiH_3)`.
(ii) `color{green}("Reactivity towards Oxygen ")` All these elements form two types of oxides : `color{red}(E_2O_3)` and `color{red}(E_2O_5)`.
● The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state.
● Their acidic character decreases down the group.
● The oxides of the type `color{red}(E_2O_3)` of nitrogen and phosphorus are purely acidic, that of arsenic and antimony amphoteric and those of bismuth is predominantly basic.
(iii) `color{green}("Reactivity towards Halogens" )` : These elements react to form two series of halides : `color{red}(EX_3)` and `color{red}(EX_5)`.
● Nitrogen does not form pentahalide due to non-availability of the `d` orbitals in its valence shell.
● Pentahalides are more covalent than trihalides.
● All the trihalides of these elements except those of nitrogen are stable.
● In case of nitrogen, only `color{red}(NF_3)` is known to be stable.
● Trihalides except `BiF_3` are predominantly covalent in nature.
(iv) `color{green}("Reactivity towards Metals ")` : All these elements react with metals to form their binary compounds exhibiting `–3` oxidation state, such as, `color{red}(Ca_3N_2)` (calcium nitride) `color{red}(Ca_3P_2)` (calcium phosphide), `color{red}(Na_3As_2)` (sodium arsenide), `color{red}(Zn_3Sb_2)` (zinc antimonide) and `color{red}(Mg_3Bi_2)` (magnesium bismuthide).